Introduction
Existing C5 inhibitor (C5i) therapies for the management of PNH, eculizumab (ecu) and ravulizumab (ravu), are typically given by regular intravenous (IV) administration (every 2 weeks [q2w] and every 8 weeks [q8w], respectively) in a hospital. Crovalimab (crova) is a novel C5i monoclonal antibody engineered with SMART-Ig recycling technology and high solubility, allowing for rapid and sustained C5 inhibition with low-volume subcutaneous (SC) injection via self-administration every 4 weeks (q4w). COMMODORE 1 (NCT04432584) and COMMODORE 2 (NCT04434092) are global, randomized, open-label, multicenter Phase III trials that investigated the efficacy and safety of crova vs ecu in C5i-experienced and C5i-naive patients (pts) with PNH, respectively (Röth EHA 2023; #S181, Scheinberg EHA 2023; #S183). Here, we report pt preference and treatment satisfaction data from COMMODORE 1 and COMMODORE 2.
Methods
In COMMODORE 1, C5i-experienced pts receiving ecu at the study start were randomized 1:1 to receive crova or ecu during a 24-week primary treatment period. Pts with the C5 polymorphism, pts previously treated with ravu or high-dose ecu, and pts ≤18 years old receiving ecu were enrolled in a descriptive arm and received crova for at least 24 weeks. In COMMODORE 2, C5i-naive pts were randomized 2:1 to receive treatment with crova or ecu during a 24-week primary treatment period. Crova was given as a weight-based tiered regimen (Liu ASH 2022; #293) that included loading doses followed by SC injection q4w maintenance dosing. Ecu was given via IV infusion q2w (900 mg per label). After completing 24 weeks of treatment, COMMODORE 1 and 2 pts randomized to crova continued crova, and those randomized to ecu switched to crova if continuing in the extension period. Crova self-administration by pts was permitted from Week 9, after training and confirmation of proficiency by a healthcare provider.
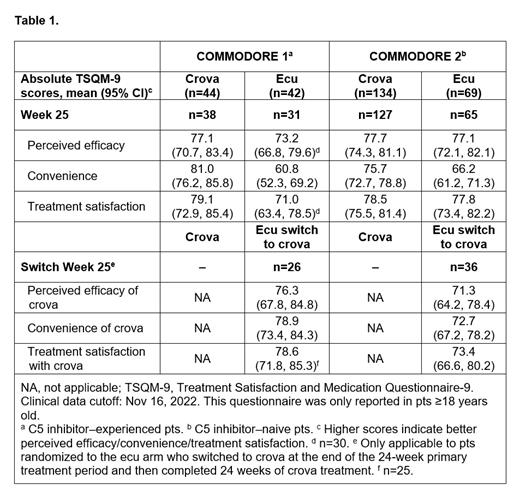
Treatment preference was assessed in adult pts using the validated Patient Preference Questionnaire after 17 weeks of crova treatment in C5i-experienced pts in all COMMODORE 1 arms and in C5i-naive pts initially randomized to ecu who switched to crova after Week 25 in COMMODORE 2. In addition, adult pts in all study arms of both studies completed the Treatment Satisfaction and Medication Questionnaire-9 (TSQM-9) at Weeks 13, 25, and 49 (or Switch Week 25). Higher TSQM-9 scores indicate better-perceived efficacy/convenience/treatment satisfaction.
Results
For the primary analysis, COMMODORE 1 randomized 45 C5i-experienced pts to crova and 44 to ecu, and enrolled 38 pts in the descriptive arm; COMMODORE 2 randomized 135 C5i-naive pts to crova and 69 to ecu. Most pts across COMMODORE 1 and COMMODORE 2 who switched from either ecu or ravu to crova preferred treatment with crova. In COMMODORE 1, 33 (85%) of the 39 pts assessed in the crova arm preferred it to ecu. Of pts randomized to ecu who switched to crova after 24 weeks, crova was preferred by 27 (96%) of the 28 pts assessed in COMMODORE 1 and 32 (84%) of the 38 pts assessed in COMMODORE 2. The top reasons selected for crova preference were that the treatment required fewer hospital visits, was easier to administer, took less time to administer, and provided a better quality of life. In the COMMODORE 1 descriptive arm, crova was preferred by 8 (57%) of the 14 ecu-experienced pts assessed (including pts with the C5 polymorphism and pts previously on high-dose ecu) and 9 (60%) of the 15 ravu-experienced pts assessed, with similar reasons provided for this preference. Further, TSQM-9 results showed that, across the randomized arms of both studies, pts had similar levels of global satisfaction with ecu and crova and perceived them as having similar efficacy, but crova was rated as more convenient (Table). In the COMMODORE 1 descriptive arm, mean scores for overall treatment satisfaction with crova ranged from 63.1 to 66.7 across the prior ravu, prior high-dose ecu, and C5 polymorphism cohorts.
Conclusions
Overall, pts in the COMMODORE 1 and COMMODORE 2 trials preferred treatment with crova to other C5i therapies. Pt perceptions of convenience around treatment frequency and mode of administration largely drove preference. With SC injection q4w and the option for self-administration outside of a supervised healthcare setting, crova has the potential to offer a new treatment option for pts with PNH that is less burdensome than existing therapies for this chronic lifelong disease.
Disclosures
Kulasekararaj:F. Hoffmann-La Roche Ltd: Consultancy, Membership on an entity's Board of Directors or advisory committees; Akari Therapeutics: Consultancy; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Samsung: Consultancy; Celgene/BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BioCryst: Consultancy; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Achillion: Consultancy; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Scheinberg:Janssen: Consultancy, Other: Scientific presentations/speaker; BMS: Other: Speaker; BioCryst: Consultancy, Research Funding; AstraZeneca: Consultancy, Other: Scientific presentations/speaker, Research Funding; Amgen: Consultancy, Other: Scientific presentations/speaker; Novartis: Consultancy, Other: Scientific presentations, Research Funding, Speakers Bureau; Pfizer: Consultancy, Other: Speaker, Research Funding; F. Hoffmann-La Roche Ltd,: Consultancy, Other: Scientific presentations, Research Funding; Viracta: Research Funding; Alexion: Consultancy, Other: Scientific presentations/speaker; Alnylam: Research Funding; AbbVie: Consultancy, Other: Speaker. Ueda:Chugai: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy; Asahi Kase: Consultancy; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SOBI: Consultancy, Honoraria. Gentile:Genentech Inc.: Current Employment. Stefani:F. Hoffmann-La Roche Ltd, Basel: Current Employment. Uguen:F. Hoffmann-La Roche Ltd, Basel: Current Employment. Roeth:Apellis Apellis Pharmaceuticals: Consultancy, Honoraria; Bioverativ: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Biocryst: Consultancy, Honoraria; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal